Comparing the COVID-19 vaccines: Pfizer, Moderna and AstraZeneca
Countries across the world are currently rolling out three main versions of the COVID-19 vaccine from Pfizer, Moderna and AstraZeneca. The first vaccines were administered in December, with plans to complete a large portion of the rollout throughout 2021.
January 15, 2021
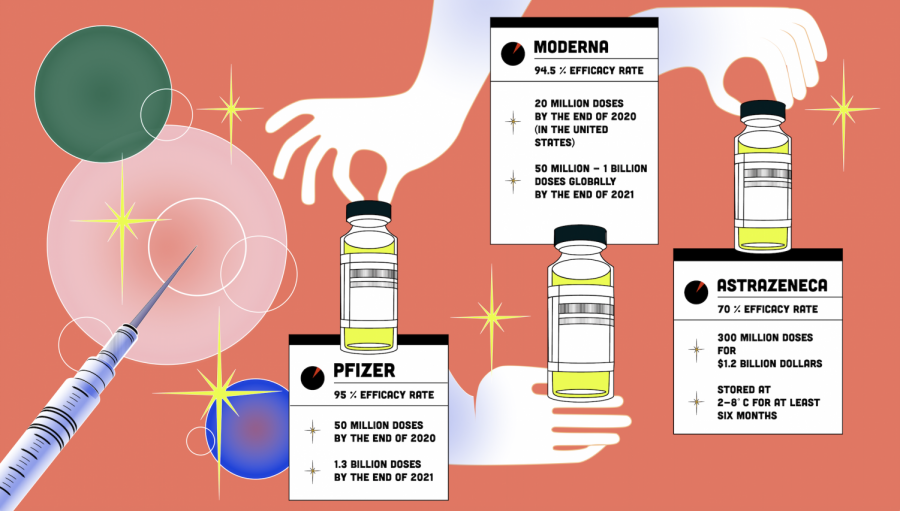
The first Pfizer-BioNTech COVID-19 vaccines were administered on Dec. 14 after being given emergency use authorization (EUA) by the Food and Drug Administration (FDA) on Dec. 11. Moderna also received EUA approval on Dec. 18 and is expected to produce 600 million doses this year. The AstraZeneca vaccine has already been approved in multiple countries, and the company plans to produce two billion doses by the end of 2021.
Pfizer:
The Pfizer-BioNTech COVID-19 vaccine contains messenger RNA (mRNA), which provides instructions for the human cells to produce spike proteins. Spike proteins are found on the coronavirus, and they will trigger an immune response, priming the body for infection. During an infection, produced antibodies prevent the spike proteins from binding to human cells, and immune cells destroy infected ones.
“The idea is [to] get the genetic information into our cells and have our cells produce the protein and elicit that immune response. That is new,” upper school biology teacher Dr. Matthew Harley said. “Previously, it was either the virus itself, whether it’s a similar virus, like cowpox, that vaccinates people against smallpox, a heat-killed virus or bacterium, or just a protein that we inject in.”
Consistent across diverse demographics the Pfizer vaccine has an efficacy of 95%. The most common side effects include mild pain at the injection site, headaches and fatigue, and they tend to be more prevalent with younger adults. Since there have not been enough trials done with young teenagers, the Pfizer vaccine has only been authorized to be used with sixteen-year-olds and older.
The vaccine needs to be stored at -70˚C, which is colder than winters in Antarctica. This is due to its mRNA-based structure.
“mRNA is the middle man between the gene of the DNA and making the actual protein. In part because it’s a single-stranded polynucleotide, it’s significantly shorter and is much more fragile, which is why to store it, we need cold temperatures, and in some cases, extremely cold temperatures,” Dr. Harley said.
Some challenges that the Pfizer vaccine faces because it needs to be kept at extremely low temperatures are transportation and storage.
“One of them has an advantage because of the refrigeration issue associated with the Pfizer vaccine. There are limitations for certain hospitals,” Anita Chetty, upper school biology teacher and science department chair said. “It puts more of a burden on smaller, rural hospitals in terms of the equipment that they have.”
On Dec. 11 the FDA authorized the vaccine, and the first shipments were received on Dec. 14 by U.S. states and territories. Pfizer expects to produce 50 million doses by the end of the year, and the vaccine must be administered in two doses given three weeks apart for full efficiency. In July, the U.S. government ordered 100 million doses for $1.95 billion, averaging to $19.50 for each dose.
“I believe that this is another example of how science is going to save us, as it has so many times,” Chetty said. “I think [the mRNA technology] is a new twist in terms of getting even closer to being able to stop the virus, through genetic manipulation.”
Moderna:
The Moderna vaccine similarly uses mRNA to give genetic material for spike proteins. However, whereas the Pfizer vaccine needs to be stored at -70˚C, Moderna’s needs to be stored at only -20˚C, the temperature of a regular freezer. This is likely because of its lipid nanoparticle technology, a type of drug delivery system that is solid at room temperature and body temperature.
The vaccine has an efficacy of 94.1% across a range of ages, genders, races, with side effects including injection site pain, fatigue and myalgia, or muscle aches.
The U.S. government ordered 100 million doses of Moderna’s vaccine for $1.525 billion, averaging to $15.25 for each dose. Another order of 100 million doses was also made for the second quarter of 2021. Since the vaccine must be administered in two doses, 28 days apart, this will be enough to cover 100 million people in the U.S.
AstraZeneca:
The AstraZeneca vaccine is based on an adenovirus, a harmless virus that can cause illnesses in chimpanzees. The adenovirus was genetically modified to contain genes from the Sars-Cov-2 proteins. Once the vaccine is injected, our bodies produce antibodies and are prepared for more severe infections.
When given as two full doses at least one month apart, the vaccine’s efficacy only reached 62%. But when given first as a half dose and followed by a full dose, it reached 90%, which is still lower than those of the Pfizer vaccine and the Moderna vaccine.
However, AstraZeneca’s vaccines can be stored and transported at just 2-8˚C, which is about the temperature of a refrigerator. Furthermore, the U.S. government ordered 300 million doses for $1.2 billion, averaging to $4 per dose. For the 90% efficacy, two doses must be taken, with the first as a half dose and the second as a full dose given at least a month later.


















![“[Building nerf blasters] became this outlet of creativity for me that hasn't been matched by anything else. The process [of] making a build complete to your desire is such a painstakingly difficult process, but I've had to learn from [the skills needed from] soldering to proper painting. There's so many different options for everything, if you think about it, it exists. The best part is [that] if it doesn't exist, you can build it yourself," Ishaan Parate said.](https://harkeraquila.com/wp-content/uploads/2022/08/DSC_8149-900x604.jpg)




![“When I came into high school, I was ready to be a follower. But DECA was a game changer for me. It helped me overcome my fear of public speaking, and it's played such a major role in who I've become today. To be able to successfully lead a chapter of 150 students, an officer team and be one of the upperclassmen I once really admired is something I'm [really] proud of,” Anvitha Tummala ('21) said.](https://harkeraquila.com/wp-content/uploads/2021/07/Screen-Shot-2021-07-25-at-9.50.05-AM-900x594.png)







![“I think getting up in the morning and having a sense of purpose [is exciting]. I think without a certain amount of drive, life is kind of obsolete and mundane, and I think having that every single day is what makes each day unique and kind of makes life exciting,” Neymika Jain (12) said.](https://harkeraquila.com/wp-content/uploads/2017/06/Screen-Shot-2017-06-03-at-4.54.16-PM.png)







![“My slogan is ‘slow feet, don’t eat, and I’m hungry.’ You need to run fast to get where you are–you aren't going to get those championships if you aren't fast,” Angel Cervantes (12) said. “I want to do well in school on my tests and in track and win championships for my team. I live by that, [and] I can do that anywhere: in the classroom or on the field.”](https://harkeraquila.com/wp-content/uploads/2018/06/DSC5146-900x601.jpg)
![“[Volleyball has] taught me how to fall correctly, and another thing it taught is that you don’t have to be the best at something to be good at it. If you just hit the ball in a smart way, then it still scores points and you’re good at it. You could be a background player and still make a much bigger impact on the team than you would think,” Anya Gert (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AnnaGert_JinTuan_HoHPhotoEdited-600x900.jpeg)

![“I'm not nearly there yet, but [my confidence has] definitely been getting better since I was pretty shy and timid coming into Harker my freshman year. I know that there's a lot of people that are really confident in what they do, and I really admire them. Everyone's so driven and that has really pushed me to kind of try to find my own place in high school and be more confident,” Alyssa Huang (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AlyssaHuang_EmilyChen_HoHPhoto-900x749.jpeg)









Larry • Jan 17, 2021 at 7:46 am
If given the option to choose a vaccine, I would select Aztrozeneca.