Debates grow over public health policy changes
by Chelsea Xie and Caden Ruan
• March 5, 2025

Pfizer authorizes booster dose for 16, 17-year-olds
by Sabrina Zhu and Alysa Suleiman
• December 14, 2021
FDA approves Pfizer’s COVID-19 vaccine
by Sabrina Zhu, STEM Editor
• August 27, 2021
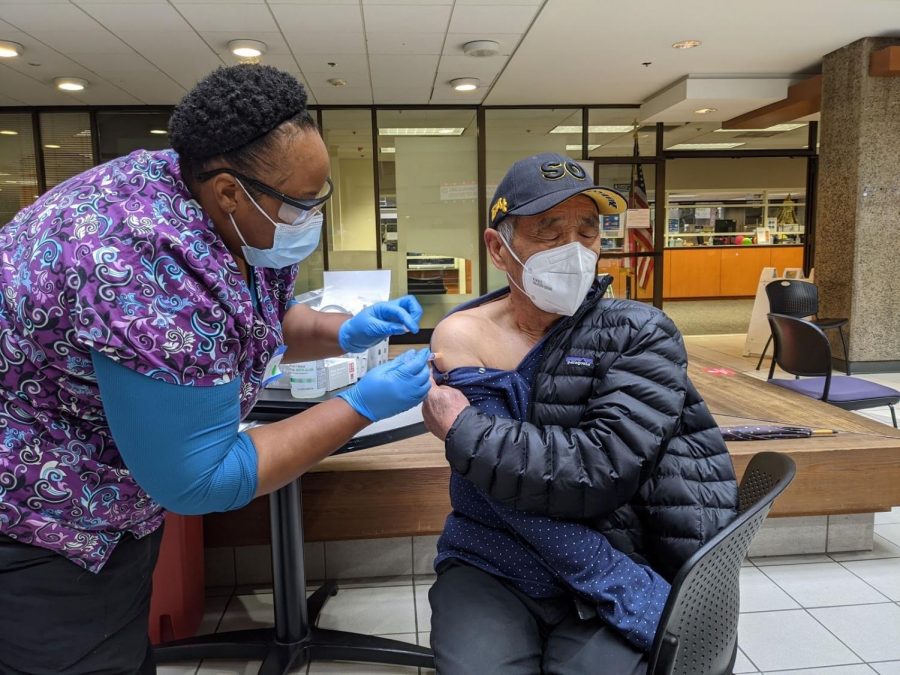
Vaccine distributions advance in the U.S., California receives 5.7 million doses
by Sabrina Zhu, Assistant STEM Editor
• February 1, 2021
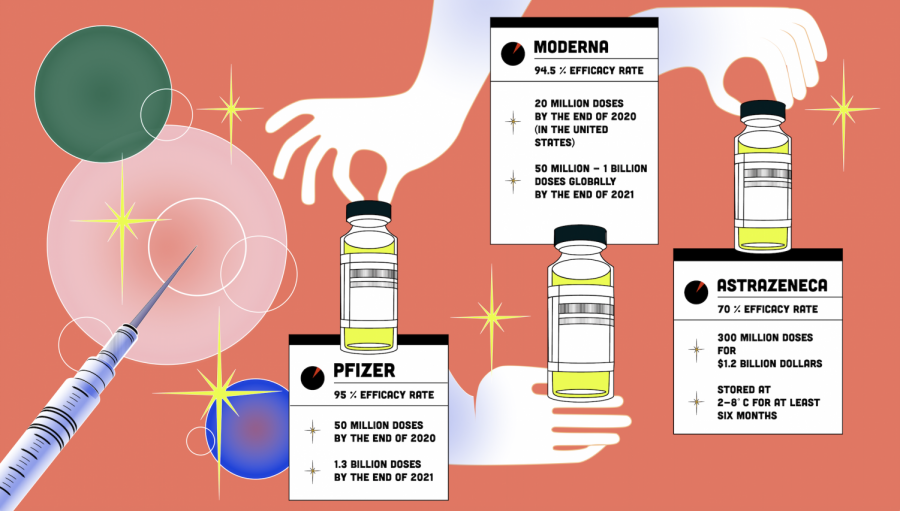
Comparing the COVID-19 vaccines: Pfizer, Moderna and AstraZeneca
by Sabrina Zhu, Assistant STEM Editor
• January 15, 2021
How the Pfizer vaccine works: A breakdown
by Sabrina Zhu and Mark Hu
• January 6, 2021
Load More Stories

by Katerina Matta, Winged Post Editor-in-Chief

by Aryana Bharali, Humans of Harker Managing Editor

by Ram Batchu, Aryana Bharali, Eva Cheng, Victor Gong, Suhani Gupta, Minal Jalil, Sam Li, Isabella Lo, Mendy Mao, Katerina Matta, Ashley Mo, Emma Milner, Young Min, Sarah Mohammed, Lily Peng, Caden Ruan, Lily Shi, Kairui Sun, Jonathan Szeto, Claire Tian, Heather Wang, Charlie Wang, Alison Yang, Chelsea Xie, Cynthia Xie, Connie Xu, Jonathan Xue, Claire Yu, Brandon Zau, and Tiffany Zhu

by Leah Krupnik, Features Editor
View this profile on InstagramHarker Aquila (@harkeraquila) • Instagram photos and videos
















![“[Building nerf blasters] became this outlet of creativity for me that hasn't been matched by anything else. The process [of] making a build complete to your desire is such a painstakingly difficult process, but I've had to learn from [the skills needed from] soldering to proper painting. There's so many different options for everything, if you think about it, it exists. The best part is [that] if it doesn't exist, you can build it yourself," Ishaan Parate said.](https://harkeraquila.com/wp-content/uploads/2022/08/DSC_8149-900x604.jpg)




![“When I came into high school, I was ready to be a follower. But DECA was a game changer for me. It helped me overcome my fear of public speaking, and it's played such a major role in who I've become today. To be able to successfully lead a chapter of 150 students, an officer team and be one of the upperclassmen I once really admired is something I'm [really] proud of,” Anvitha Tummala ('21) said.](https://harkeraquila.com/wp-content/uploads/2021/07/Screen-Shot-2021-07-25-at-9.50.05-AM-900x594.png)







![“I think getting up in the morning and having a sense of purpose [is exciting]. I think without a certain amount of drive, life is kind of obsolete and mundane, and I think having that every single day is what makes each day unique and kind of makes life exciting,” Neymika Jain (12) said.](https://harkeraquila.com/wp-content/uploads/2017/06/Screen-Shot-2017-06-03-at-4.54.16-PM.png)







![“My slogan is ‘slow feet, don’t eat, and I’m hungry.’ You need to run fast to get where you are–you aren't going to get those championships if you aren't fast,” Angel Cervantes (12) said. “I want to do well in school on my tests and in track and win championships for my team. I live by that, [and] I can do that anywhere: in the classroom or on the field.”](https://harkeraquila.com/wp-content/uploads/2018/06/DSC5146-900x601.jpg)
![“[Volleyball has] taught me how to fall correctly, and another thing it taught is that you don’t have to be the best at something to be good at it. If you just hit the ball in a smart way, then it still scores points and you’re good at it. You could be a background player and still make a much bigger impact on the team than you would think,” Anya Gert (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AnnaGert_JinTuan_HoHPhotoEdited-600x900.jpeg)

![“I'm not nearly there yet, but [my confidence has] definitely been getting better since I was pretty shy and timid coming into Harker my freshman year. I know that there's a lot of people that are really confident in what they do, and I really admire them. Everyone's so driven and that has really pushed me to kind of try to find my own place in high school and be more confident,” Alyssa Huang (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AlyssaHuang_EmilyChen_HoHPhoto-900x749.jpeg)