Scientists discover new elements
January 31, 2016
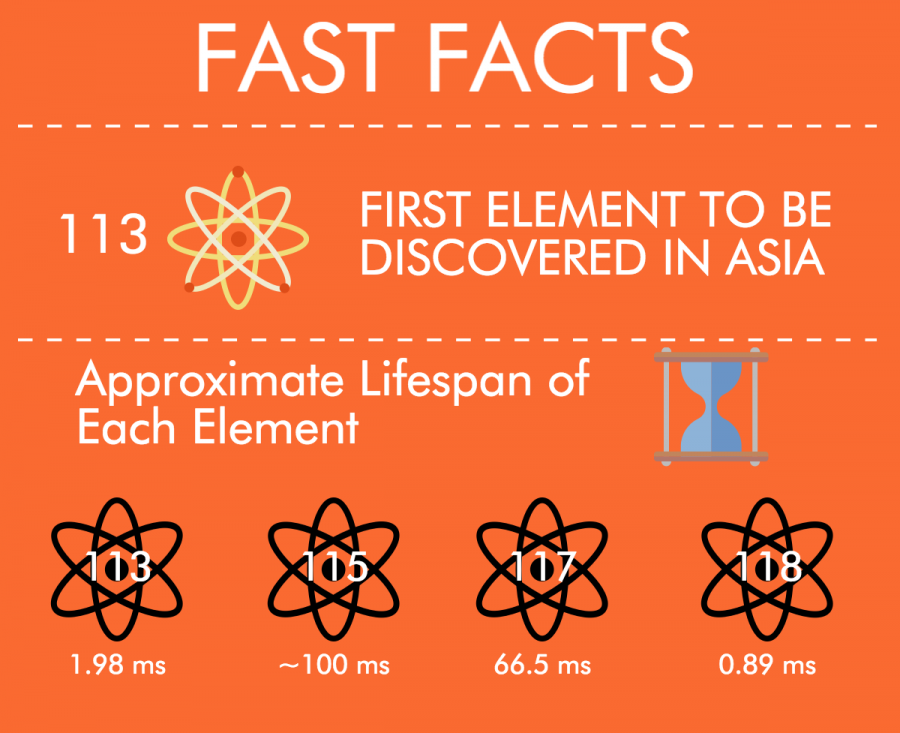
The International Union of Pure and Applied Chemistry (IUPAC) confirmed of the discovery of four new elements with atomic numbers 113, 115, 117 and 118 on Dec 30.
These new discoveries are “artificial elements,” meaning that they do not occur naturally and quickly decay into more stable elements.
“These [elements] come from particle physics experiments when they smash two elements,” chemistry teacher Dr. Mala Raghavan said. “It’s really fascinating to see if these elements even can be formed.”
Artificial elements like Lawrencium, Flerovium and the four new elements are very unstable and decompose into other elements within fractions of a second. Because of the time sensitivity of these elements, they have little practical value and will not impact the school’s chemistry curriculum.
Chemistry teacher Smriti Koodanjeri explained why scientists strive to discover new elements despite their historic instability.
“There’s a belief that at some point we’ll come across a stable element,” Koodanjeri said. “Also, we don’t know what else we might discover while studying these new elements; we might discover something that’s useful elsewhere.”
Element 113 (working name Ununtrium) was discovered at Rikagaku Kenkyūsho (RIKEN), a large Japanese research institution.
Ununtrium is the first element ever to be discovered by a group in Asia. Although Japanese chemist Masataka Ogawa claimed to have discovered element 43 (Technetium) in 1909, naming it “Nipponium (Np)” after Japan (Nippōn in Japanese), other researchers were unable to reproduce his work. In reality, he had discovered element 75 (Rhenium) and the abbreviation “Np” was later appropriated for Neptunium.
Element 115 (Ununpentium) and Element 117 (Ununseptium) were discovered in a collaboration between scientists from the Joint Institute for Nuclear Research (JINR) in Russia, the Lawrence Livermore National Laboratory (LLNL) in California and the Oak Ridge National Laboratory (ORNL) in Tennessee.
JINR and LLNL also worked together to discover Element 118 (Ununoctium).
Excepting RIKEN, all three of the discovering institutions have named and discovered artificial elements before. JINR discovered element 116, named Livermorium in Lawrence Livermore Laboratory’s honor; the JINR discovered element 114, named Flerovium after its Flerov Laboratory, which itself is named after Russian physicist Gregory Flyorov; and ORNL discovered element 61, or Promethium, named after the bringer of fire in Greek mythology.
These four elements will be officially named by the labs that made the discoveries. Proposed names will be greenlighted by the IUPAC, which states that “elements can be named after a mythological concept, a mineral, a place or country, a property or a scientist.”
Students expressed mixed opinions on how these new elements will shape science.
Michael Wang (9) does not believe the scientific community will be greatly impacted by these discoveries.
“These elements were already predicted, so I don’t think the scientific community will be much affected other than the fact that another prediction has come true,” Michael said.
Other students interpreted these discoveries as indicative of humanity’s high scientific achievements.
“I think currently the application is still limited, but the fact that we have the ability to make these discoveries shows that we’ve been progressing and our technology is advanced enough to really start exploring our world in a more calculated manner,” David Zhu (11) said.
Although this progress is exciting, scientists strive to make more discoveries in the future.


















![“[Building nerf blasters] became this outlet of creativity for me that hasn't been matched by anything else. The process [of] making a build complete to your desire is such a painstakingly difficult process, but I've had to learn from [the skills needed from] soldering to proper painting. There's so many different options for everything, if you think about it, it exists. The best part is [that] if it doesn't exist, you can build it yourself," Ishaan Parate said.](https://harkeraquila.com/wp-content/uploads/2022/08/DSC_8149-900x604.jpg)




![“When I came into high school, I was ready to be a follower. But DECA was a game changer for me. It helped me overcome my fear of public speaking, and it's played such a major role in who I've become today. To be able to successfully lead a chapter of 150 students, an officer team and be one of the upperclassmen I once really admired is something I'm [really] proud of,” Anvitha Tummala ('21) said.](https://harkeraquila.com/wp-content/uploads/2021/07/Screen-Shot-2021-07-25-at-9.50.05-AM-900x594.png)







![“I think getting up in the morning and having a sense of purpose [is exciting]. I think without a certain amount of drive, life is kind of obsolete and mundane, and I think having that every single day is what makes each day unique and kind of makes life exciting,” Neymika Jain (12) said.](https://harkeraquila.com/wp-content/uploads/2017/06/Screen-Shot-2017-06-03-at-4.54.16-PM.png)







![“My slogan is ‘slow feet, don’t eat, and I’m hungry.’ You need to run fast to get where you are–you aren't going to get those championships if you aren't fast,” Angel Cervantes (12) said. “I want to do well in school on my tests and in track and win championships for my team. I live by that, [and] I can do that anywhere: in the classroom or on the field.”](https://harkeraquila.com/wp-content/uploads/2018/06/DSC5146-900x601.jpg)
![“[Volleyball has] taught me how to fall correctly, and another thing it taught is that you don’t have to be the best at something to be good at it. If you just hit the ball in a smart way, then it still scores points and you’re good at it. You could be a background player and still make a much bigger impact on the team than you would think,” Anya Gert (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AnnaGert_JinTuan_HoHPhotoEdited-600x900.jpeg)

![“I'm not nearly there yet, but [my confidence has] definitely been getting better since I was pretty shy and timid coming into Harker my freshman year. I know that there's a lot of people that are really confident in what they do, and I really admire them. Everyone's so driven and that has really pushed me to kind of try to find my own place in high school and be more confident,” Alyssa Huang (’20) said.](https://harkeraquila.com/wp-content/uploads/2020/06/AlyssaHuang_EmilyChen_HoHPhoto-900x749.jpeg)