A New Era for Gene Editing?
January 9, 2017
Following the advent of CRISPR and other strides in genomic technologies, genetic engineering has emerged as one of the most controversial topics in the modern scientific community.
Gene editing is founded upon deceptively simple premises. A person’s genome, or collection of genes, encodes for unique characteristics such as height, eye color and intelligence. It also accounts for negative traits, such as diseases and disorders. (Some traits are acquired from the environment – the extent of the influence of nature and nurture is hotly debated even to this day.) Accordingly, edits made to an individual’s genome will alter their inherited traits. Anything encoded for by genes can be changed by editing the encoding gene.
While scientists have explored the idea of gene editing for decades, previous methods of editing the human genome were unreliable and costly, rendering any actual implementation theoretical.
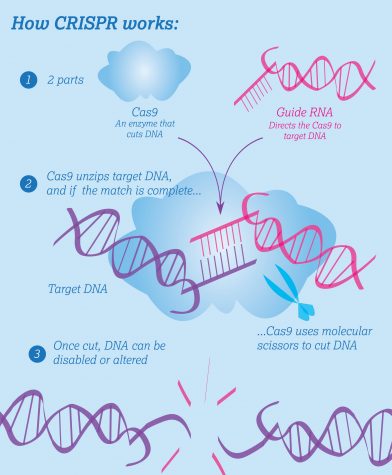
Recently, however, researchers have developed faster and more reliable gene editing techniques. The CRISPR-Cas9 method, which uses the bacterial Cas9 enzyme to cut out a targeted gene in a DNA sequence and allows researchers to add new ones, has recently risen into the scientific spotlight. CRISPR’s accuracy, simple construction and low cost make it the most promising option for making genetic engineering viable. This development has not come without its warranted measure of caution – with gene editing becoming a reality, discussion about whether and how it should be used has resurfaced both within the scientific community and beyond.
“There has definitely been a lot of conversation and debate within the CRISPR community about the responsibility that we have as researchers for how we use these tools and what kind of implications and impacts that it will have on society at large,” said Marie La Russa, a Ph.D. candidate at the Stanford Stanley Qi lab, which conducts extensive research with CRISPR. “I think this question cannot and should not be answered solely by scientists – it needs to be something that engages the community, engages global government, ethicists, people informed and interested citizens to kind of come up with
ideas for how we should move forward.”
One of the most exciting potential applications of gene editing is gene therapy, a theorized technique to treat genetic diseases by replacing defective genes with their “normal” versions.
Genetic diseases, which are caused by mutated forms of genes, represent some of the most debilitating diseases known to physicians, such as Huntington’s chorea, which causes neurological degeneration and uncontrollable motor movements, and cystic fibrosis, which causes mucosal buildups in the respiratory tracts and immense difficulty with breathing. A growing body of evidence further suggests that genetics can play a role in causing seemingly non-genetic diseases as well, such as Alzheimer’s disease and breast cancer.
As of now, physicians and doctors have not found ways to cure genetic diseases. Unlike other diseases, which have an interactable agent, an individual’s own genome causes genetic diseases, making the patient and the disease inseparable. While the use of medication can mitigate some genetic diseases, these treatments are reactive, expensive and incomplete.
In theory, there an obvious and simple way to cure genetic diseases is to replace the mutated gene with a copy of the “normal” gene. Using this method, physicians could completely cure any genetic disease – provided that the disease-causing mutation is known.
If proven effective, gene editing could be used to cure genetic diseases, representing a massive boon in improving public health and quality of life. No longer would parents suffer the ravages of incurable congenital disorders such as Tay-Sachs disease, which causes the degeneration of nerve cells in the brain, usually killing victims before the age of four.
While gene therapy can be used to cure genetic diseases, it could also be used to lessen the impacts of less severe conditions, such as asthma or myopia. It is unclear whether these conditions constitute necessary gene edits – while they clearly can negatively impact quality of life, they aren’t life-threatening.
“It’s hard to say what defines whether or not we should make an edit,” said Rajiv Movva (‘18), who conducted research with CRISPR over the summer. “I think that there is a bright line that has to be drawn as to what is something that has a detrimental effect on a human’s quality of life, and what doesn’t. That’s definitely a slippery slope that we’ll have to tread lightly on, but I think that for certain things like, for example Alzheimer’s and Parkinson’s, it’s very fair to say that the embryo and all of its possible descendants will be better off without this crippling disease.”
Taken further, even beyond curing genetic diseases, gene edits could be used to enhance other characteristics, such as intelligence and height, or even purely cosmetic ones, such as hair color. Provided enough knowledge about the genome, it could become possible to edit a fetus’s genome to enhance their physical characteristics, such as beauty, strength and intelligence. Some have even proposed that allowing widespread gene editing will result in babies who have been tailored to their parents’ tastes – “designer babies.”
The American public has reflected these doubts over the appropriateness of these enhancement edits as compared to strictly pathological ones. According to a Pew Research Center poll in 2014, while 46% of American adults responded that modifying a baby’s genome to reduce their risk of serious diseases was an appropriate use of medical technology, only 15% found it appropriate to modify for increased intelligence, with 83% responding that it was overtly “taking medical advances too far.”
“[Something] that has been addressed more in science fiction than in science, there is another bigger concern [that] by opening the door to addressing genetic diseases, we are also now opening the door to other reasons for modifying the genome, less health oriented,” Spenner said.
The primary appeal of gene editing comes from the idea of being able to cure a debilitating disease or improve a person’s life in a way that could not be done otherwise.
“If you’re having a kid, and your doctor comes to you and says, ‘Well, we can do this procedure that will maybe not ensure, but with some confidence, pretty certain[ly] will prevent a disease or make him or her healthier over the course of [their] lifespan’ – if that was all a new parent was told, I think a lot of parents would be in favor of doing it,” research teacher Chris Spenner said.
Gene editing is not to be taken lightly – changes made to an individual’s genome live with them forever. Furthermore, changes made to individual’s sex cells will be passed on to their descendants, permanently altering the lives of their offspring as well – edits done after cell line differentiation could avoid this, but edits made before would be heritable.
“We really don’t understand how the genome works,” Spenner said. “We’re still learning about epigenomics and all these other subtleties – more than just subtleties, these other complications – that suggest that just knowing the primary sequence of the gene code does not show entirely what’s going to happen, and that also we don’t know how to predict how genetic modifications might interact with the natural environment later on.”
Though some argue that using gene editing to produce more intelligent, strong or attractive children could increase the potential of the human race, others believe that access to cosmetic gene alterations could further stratify people into social classes based off of intelligence, talent, strength or appearance. This outcome has most prominently been depicted in Aldous Huxley’s dystopian futuristic novel, Brave New World, in which the the government modifies human embryos to be less or more intelligent depending on their social caste, concretely and inexorably committing them to their ordained future. Permitting parents to use gene editing for cosmetic purposes could further exacerbate extant socioeconomic differences due to unequal access to the technology, as only those who can afford to genetically modify their children could do so.
“Right now there’s clearly some relevance with technology we already have in the world, like access to better technology or medical treatments or cosmetic surgeries, for example,” Amy Jin (‘18), who participates in discussion about gene editing’s ethics, said. “That is already something that some people cannot afford while others can. With gene editing, that would be the same thing because the wealthy and the powerful would have the means of getting access to this, even if it’s illegal.”
Allowing widespread use of gene editing techniques for cosmetic purposes can also lead to abuse and misuse of these methods. Although scientists who are familiar with this technology may be able to use it safely, those who are inexperienced or have malicious intentions can use gene editing incorrectly and thus negatively impact the person receiving the treatment.
“Once this technology is introduced and is more developed, then those who want to abuse it will definitely try to get their hands on this technology and it also would empower the wealthy while marginalizing the poor,” Amy said.
In addition to ethical and moral concerns about the use of gene editing, some question whether current gene editing techniques are safe and reliable enough to be used on humans. Even if society accepted gene editing as ethical, CRISPR has a long way to go before it can be considered safe for therapeutic use. Several technical complications challenge the scientific feasibility of gene editing.
Scientists have discovered that in some cases, gene editing techniques like CRISPR inadvertently modify sequences besides those being targeted. These unintended edits, known as “off-target gene editing,” could cause detrimental and potentially life-threatening changes to the organism being modified.
“In terms of precision, we know the exact base pair that [the Cas9 enzyme’s] going to cut if we direct it to our on-target site, but if we give it a sequence to direct it to a target site, how often will it go to that site rather than cutting or affecting other sites that might have a similar sequence?” La Russa said. “We know the sequence of the whole genome, so we can design a guide that can be reasonably very highly specific just based off of knowing it’s not going to target any other sequence in the genome. It can be very precise, but I think if we want to use it for therapeutic applications, there definitely needs to be a lot more work done to characterise specific target sites and how often the Cas9 will cut or bind other sites just to make sure that we’re only affecting the genes that we want to when we put the CRISPR system into cells.”
Even if an edit is successfully made without changing any other genes, editing a gene that impacts a certain trait or condition may inadvertently affect other characteristics. Many genes are pleiotropic, meaning that they affect multiple seemingly unrelated traits.
An edit meant to improve a person’s quality of life could inadvertently detrimentally affect the person in unforeseen ways.
“We do need a clearer understanding of the effects of genetics on disease traits, and right now it’s hard to know when we have a good enough understanding of the disease,” Amy said. “As of right now, we wouldn’t be able to carry through with gene editing because we don’t know the potential unintended effects that perhaps altering or splicing DNA could do when we’re trying to cure a disease.”
Our current incomplete knowledge of the complex genetic interactions within the human body makes it impossible to determine what editing a certain gene will ultimately do to the person being treated.
“We have not yet used these kinds of technologies and we have not seen [their full effects,]” Spenner said. “It would take a full lifespan to know for sure what the downstream effects are – it’s a few years into the technology, it’s way too early.”
Furthermore, it is usually rare that a particular allele is completely negative, or completely positive – some traits we currently consider “diseases” can have beneficial side effects. It is sometimes unclear as to what constitutes a disease, both in the scientific sense but also in the philosophical one.
For instance, take the genetic disorder sickle cell anemia. Those who have sickle cell anemia produce malformed hemoglobin, causing their blood cells to ineffectively carry oxygen and clot.
While the full-blown disease is debilitating, those with merely the Sickle cell trait, or just a single copy of the sickle cell anemia gene, actually have an advantage over “normal” blood cell people in certain circumstances. Sickle-shaped blood cells are resistant to infection by the malaria parasite, meaning that sickle cell trait individuals are more resistant to malaria. (It is observed that the sickle cell trait is more prevalent in areas where malaria is common)
Sometimes, the ambiguity lies not with the trait having multiple positive and negative effects, but with whether the trait’s one effect should be characterised as positive or negative.
“Another great example is Asperger[’s syndrome],” Rajiv said. “We see with Asperger’s that patients often have decreased cognitive ability in the EQ [emotional quotient, a test of “emotional intelligence”] sense, but often times they’re very talented in mathematics and other patterned subjects. This goes back to the question, ‘Should we characterize [diseases] before [gene editing] enters medical practice?’ [. . .] There’s always a possibility for error, that we will miss something, but as much as we can we should try to be exhaustive in our scientific study.”
These ethical issues aside, technical difficulties also hinder the implementation of gene editing procedures.
Some traits and diseases are polygenic, meaning that they are actually the product of not one gene, but multiple different genes at different places on the genome. This represents a significant obstacle to gene therapy – until the disease is sufficiently well-characterized, we may not currently recognize all of the contributing genes and lack the knowledge to properly edit all of the necessary sites and actually cure the disease.
Knowledge about the mutations which cause the disease varies greatly between diseases. For instance, sickle cell anemia is caused by a single point mutation in the hemoglobin gene – the switch of just one nucleotide base for another.
But other diseases, such as type 1 and type 2 diabetes may depend on multiple genes and even environmental factors. In these cases, to eliminate or modify the trait would be more difficult than simply altering or removing a certain sequence: gene-editing techniques are currently unable to accommodate for complex polygenic diseases or characteristics.
“Too many traits are controlled by hundreds or thousands of genes, like intelligence and even eye color or hair, or skin color is polygenic,” Amy said.
Altogether, the prospect of gene editing should be considered with utmost gravity and caution. Gene editing is confounded with multiple questions as to the extent of its ethical use, its scientific viability, and the limits of our current knowledge as to its validity.
A consensus must ultimately be reached about the use of gene editing – what it will be considered appropriate for, and in which circumstances. Within the scientific community, biologists from across the globe called for a moratorium on editing the human genome in 2015. But a proper decision about gene editing should engage people outside of the scientific community.
“There have already been factions in the scientific community that have fallen in different areas as far as the ethics go.The scientific community is already having their own internal debate, and that is ongoing,” Spenner said.
“I think that [the debate] has to be eventually opened up to not just policy makers, but to the public as well. I think good science needs to be democratic in that sense and transparent. I always worry about the level of science education, and how informed the decisions will be that are made later on, but I think it will be far better to have an official forum for policy makers and for the public rather than to have discussions happen or decisions being made under the table and without transparency,” he said.
Regardless of the decisions made, gene editing will drastically shape the nature of human disease and medicine in the years to come. Like the discoveries of electricity and nuclear fission, gene editing will represent a landmark technology in science history and change society forever – for better or for worse.